

Defects of the limb skeleton are among the most frequent human congenital malformations. In the embryonic limb buds of humans and other vertebrates the sites of future skeletal elements are marked by the entry of precartilage mesenchymal cells into tight aggregates or condensations. Shortly thereafter, the cells within these condensations differentiate into cartilage, establishing the primordia for the bony skeleton. Our work is directed towards understanding the dynamical process by which the interactions of limb bud cells with various factors they produce results in a series of articulated, well-arranged rods and nodules of cartilage.
Precartilage condensation reflects changed cell-extracellular matrix and cell-cell interactions, and leads to cytoskeletal-linked changes in cell shape. Once condensations have formed, a cascade of signals are entrained that ultimately results in the differentiation of the mesenchymal foci into cartilage. This signaling involves prostaglandin release, elevation of intracellular cAMP, and phosphorylation of nuclear proteins by protein kinase A-dependent and -independent mechanisms.
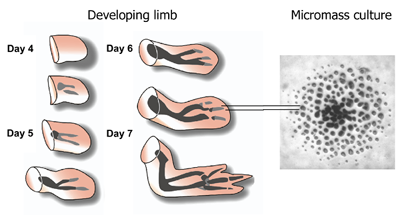
Because limb bud mesenchyme undergoes condensation and chondrogenesis (cartilage differentiation) in vitro according to a timetable similar to that occurring in vivo, the events leading to cartilage differentiation can be readily studied in culture using cells derived from the limb buds of chicken or mouse embryos. Chicken limb bud mesenchyme in serum-free culture forms a continuous sheet of cartilage or a set of isolated nodules of cartilage, depending upon whether it is derived from the wing or leg bud (see Figure). Therefore this tissue culture model can also be used to study of cartilage pattern formation. Questions raised by this set of phenomena concern the identity of factors that determine where and when foci of condensation are initiated and how the expansion of cell condensations are limited. It is also of great interest to determine what cytoplasmic and nuclear signaling events are responsible for transforming mesenchymal cells into cartilage cells once condensations have formed
The extracellular matrix molecule fibronectin is an important early mediator of the condensation process. Our current work involves identifying secreted factors of the limb mesenchyme, such as TGF-β, that regulate the spatiotemporal production of fibronectin. We have also been studying the amino acid sequence determinants of fibronectin critical for its interaction with the mesenchymal cell surface. Another recent set of studies concerns the mechanism by which the intracellular elevation of cAMP at sites of precartilage condensation induces changes in gene expression associated with cartilage differentiation. We are also studying the role of hnRNP A1, a developmentally-regulated splicing factor that controls alternative splicing of mesenchymal FGF receptors, a key step in skeletal pattern formation.
Selected references:
Newman, S. A., and Frisch, H. L. (1979) Dynamics of skeletal pattern formation in developing chick limb. Science 205, 662-668. (PDF)
Frenz, D. A., Akiyama, S. K., Paulsen, D. F., and Newman, S. A. (1989) Latex beads as probes of cell surface-extracellular matrix interactions during chondrogenesis: evidence for a role for amino-terminal heparin-binding domain of fibronectin. Dev Biol 136, 87-96. (PDF)
Frenz, D. A., Jaikaria, N. S., and Newman, S. A. (1989) The mechanism of precartilage mesenchymal condensation: a major role for interaction of the cell surface with the amino-terminal heparin-binding domain of fibronectin. Dev Biol 136, 97-103. (PDF)
Leonard, C. M., Fuld, H. M., Frenz, D. A., Downie, S. A., Massagué, J., and Newman, S. A. (1991) Role of transforming growth factor-b in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenous TGF-b and evidence for endogenous TGF-b-like activity. Dev Biol 145, 99-109. (PDF)
Downie, S. A., and Newman, S. A. (1994) Morphogenetic differences between fore and hind limb precartilage mesenchyme: relation to mechanisms of skeletal pattern formation. Dev Biol 162, 195-208. (PDF)
Downie, S. A., and Newman, S. A. (1995) Different roles for fibronectin in the generation of fore and hind limb precartilage condensations. Dev Biol 172, 519-530. (PDF)
Newman, S. A. (1996) Sticky fingers: Hox genes and cell adhesion in vertebrate limb development. BioEssays 18, 171-174. (PDF)
Zhang, Q., Carr, D. W., Lerea, K. M., Scott, J. D., and Newman, S. A. (1996) Nuclear localization of type II cAMP-dependent protein kinase during limb cartilage differentiation is associated with a novel developmentally-regulated A-kinase anchoring protein. Dev Biol 176, 51-61. (PDF)
Moftah, M. Z., Downie, S. A., Bronstein, N. B., Mezentseva, N., Pu, J., Maher, P. A., and Newman, S. A. (2002) Ectodermal FGFs induce perinodular inhibition of limb chondrogenesis in vitro and in vivo via FGF receptor 2. Dev Biol 249, 270-82. (PDF)
Bronstein, N. B., Kishore, R., Ismail, Z., Zhang, Q., Taylor, T., and Newman, S. A. (2003) cDNA cloning and spatiotemporal expression during avian embryogenesis of hnRNP A1, a regulatory factor in alternative splicing. Gene Expr Patterns 3, 285-95. (PDF)
Kiskowski, M. A., Alber, M. S., Thomas, G. L., Glazier, J. A., Bronstein, N. B., Pu, J., and Newman, S. A. (2004) Interplay between activator-inhibitor coupling and cell-matrix adhesion in a cellular automaton model for chondrogenic patterning. Dev Biol 271, 372-87. (PDF)
Hentschel, H. G., Glimm, T., Glazier, J. A., and Newman, S. A. (2004) Dynamical mechanisms for skeletal pattern formation in the vertebrate limb. Proc R Soc Lond B Biol Sci 271, 1713-1722. (PDF)
Chaturvedi, R., Huang, C., Kazmierczak, B., Schneider, T., Izaguirre, J. A., Glimm, T., Hentschel, H. G. E., Glazier, J. A., Newman, S. A., and Alber, M. (2005) On multiscale approaches to three-dimensional modeling of morphogenesis. J R Soc Interface 2, 237?253. (PDF)
Cickovski, T., Huang, C., Chaturvedi, R., Glimm, T., Hentschel, H. G. E., Alber, M., Glazier, J. A., Newman, S. A., and Izaguirre, J. A. (2005) A framework for three-dimensional simulation of morphogenesis. IEEE/ACM Trans Computat Biol Bioinformatics 2, 273-288. (PDF)
Newman, S. A. (2007) The Turing mechanism in vertebrate limb patterning. Nat Rev Mol Cell Biol 8, http://www.nature.com/nrm/journal/v8/n6/full/nrm1830-c1.html. (PDF)
Christley, S., Alber, M. S., and Newman, S. A. (2007) Patterns of mesenchymal condensation in a multiscale, discrete stochastic model. PLoS Comput Biol 3, (e76) 0743-0753. (link)
Newman, S. A., and Bhat, R. (2007) Activator-inhibitor dynamics of vertebrate limb pattern formation. Birth Defects Res C Embryo Today 81, 305-319. (PDF)
Newman, S. A., Christley, S., Glimm, T., Hentschel, H. G., Kazmierczak, B., Zhang, Y. T., Zhu, J., and Alber, M. (2008) Multiscale models for vertebrate limb development. Curr Top Dev Biol 81, 311-40. (link)
Alber, M., Glimm, T., Hentschel, H. G., Kazmierczak, B., Zhang, Y. T., Zhu, J., and Newman, S. A. (2008) The morphostatic limit for a model of skeletal pattern formation in the vertebrate limb. Bull Math Biol 70, 460-83. (PDF)
Damon, B. J., Mezentseva, N. V., Kumaratilake, J. S., Forgacs, G., and Newman, S. A. (2008) Limb bud and flank mesoderm have distinct "physical phenotypes" that may contribute to limb budding. Dev Biol 321, 319-30. (PDF)
Zhu, J., Zhang, Y.-T., Newman, S. A., Alber, M. S. (2009) A discontinuous Galerkin finite element model on moving grids for vertebrate limb pattern formation. Math Model Nat Phenom 4, 131-148. (PDF)
Zhu, J., Zhang, Y. T., Alber, M. S., Newman, S. A. (2010) Bare bones pattern formation: a core regulatory network in varying geometries reproduces major features of vertebrate limb development and evolution. PLoS One 5, e10892. (link)
Bhat, R., Lerea, K. M., Peng, H., Kaltner, H., Gabius, H. J., Newman, S. A. (2011) A regulatory network of two galectins mediates the earliest steps of avian limb skeletal morphogenesis. BMC Dev Biol 11, 6 (link)
Glimm, T., Zhang, J., Shen, Y. Q., Newman, S. A. (2012) Reaction-diffusion systems and external morphogen gradients: the two-dimensional case, with an application to skeletal pattern formation. Bull Math Biol 74, 666-87 (PDF)
Zhang, Y. T., Alber, M. S., Newman, S. A. (2013) Mathematical modeling of vertebrate limb development. Math Biosci 243, 1-17. (PDF)
Glimm, T., Bhat, R., Newman, S. A. (2014) Modeling the morphodynamic galectin patterning network of the developing avian limb skeleton. J Theor Biol 346, 86-108. (PDF)
Bhat, R., Chakraborty, M., Mian, I. S., Newman, S. A. (2014) Structural divergence in vertebrate phylogeny of a duplicated prototype galectin. Genome Biol Evol 6, 2721-2730. (link)
Bhat, R., Chakraborty, M., Glimm, T., Stewart, T. A., Newman, S. A. (2016) Deep phylogenomics of a tandem-repeat galectin regulating appendicular skeletal pattern formation. BMC Evol Biol 16, 162. (link)